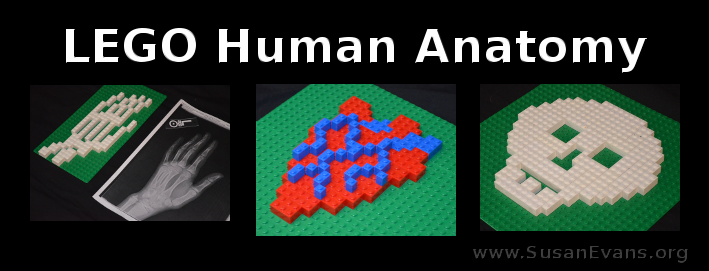
Why not make some LEGO human anatomy models? You can start with the skeletal system, with the bones of the body. One of my sons got into a scooter accident last summer and had to go to the emergency room. Here is his hand x-ray, where we found out that there was only a tiny fracture, not bad enough to get a cast. He said that the pain hurt like the dickens. But alas, there was nothing we could do.
When we started our human anatomy unit study this fall, the first body system we studied was the skeletal system. My son whipped out his hand x-ray and made a LEGO model of his hand with white LEGOs on a green base. It turned out looking pretty cool.
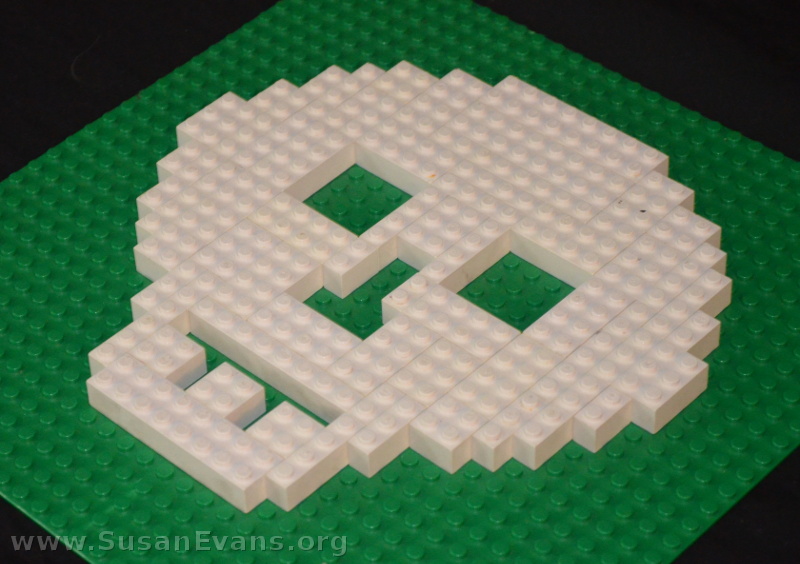
Next he made a skull. No, he didn’t get an x-ray of his skull when he cracked his head on the cement from flipping off his scooter. He didn’t ever get a concussion, so the doctors didn’t bother getting an x-ray of his skull. Instead, my son looked at a picture of a skull (perhaps in the hand of Hamlet), and made a LEGO model of the skull.
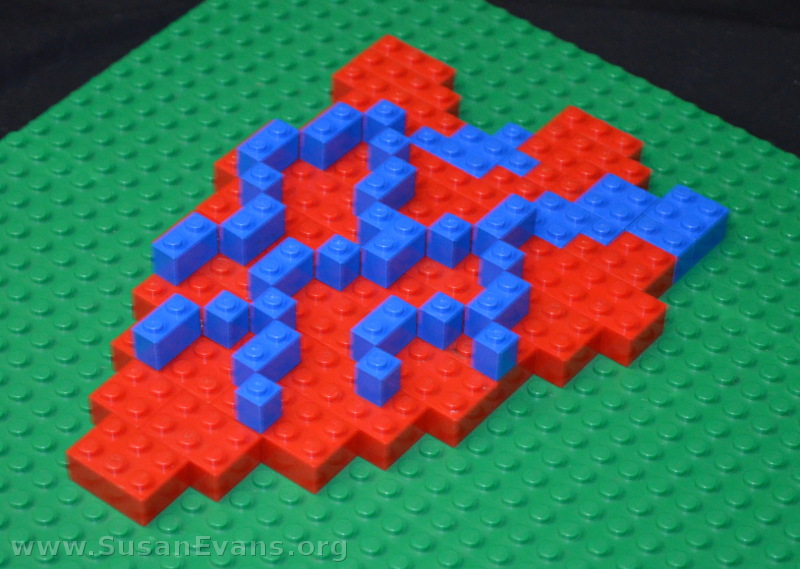
Not to remain in the skeletal system forever, my son decided to make a model of the human heart. He looked at a diagram of the human heart and used red LEGOs to form the general shape. Then he used blue LEGOs for the arteries.
So there you have it. LEGO human anatomy, ladies and gentlemen.
If you want more hands-on activities for human anatomy, join the Unit Study Treasure Vault!