 This post contains affiliate links. I was compensated for my work in writing this post.
This post contains affiliate links. I was compensated for my work in writing this post.
Today I will show you how to build atomic models with styrofoam balls and with candy. You will see two very different models, and yet the protons, neutrons, and electrons are in the same locations. My kids are using the book Christian Kids Explore Chemistry by Bright Ideas Press. We are learning the composition of an atom.
In the center of the atom is the nucleus. The nucleus is composed of protons and neutrons. The electrons are much smaller, and they spin in a cloud around the nucleus.
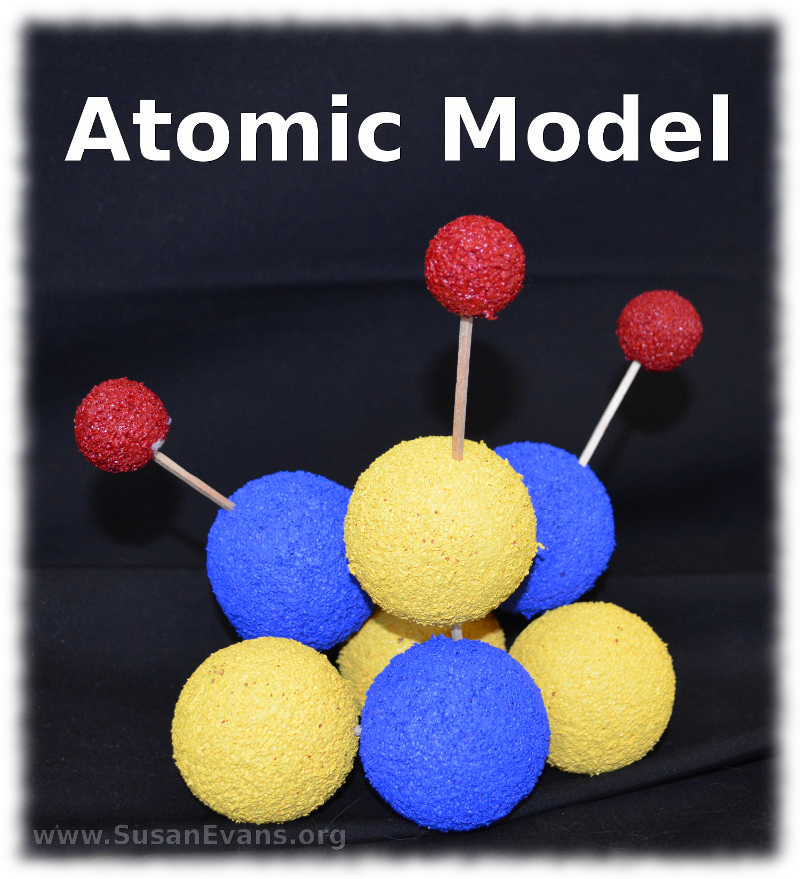
When making a styrofoam model of a Lithium atom, you need 7 two-inch styrofoam balls and 3 one-inch styrofoam balls. Lithium has 3 protons (which you paint blue), 4 neutrons (which you paint yellow), and 3 electrons (which you paint red). We used acrylic paint because it’s brighter than tempera paint, and it sinks into the texture of the styrofoam. After painting the styrofoam balls, let them dry overnight.
The next day stab the yellow and blue balls together with toothpicks until the balls are touching. This is the nucleus. Now stab the three red balls further away with toothpicks. These are electrons. You have completed your atomic model of Lithium.
 Now let’s make a candy atom just for fun, so that you can see a variety of atomic models. You could also use play doh or clay to make atomic models. But candy tastes good, so let’s use candy.
Now let’s make a candy atom just for fun, so that you can see a variety of atomic models. You could also use play doh or clay to make atomic models. But candy tastes good, so let’s use candy.
We are about to make an Oxygen atom. Grab 8 green protons and 8 red neutrons. These are gum drops, and if they stick together, fine. If the nucleus isn’t sticking together, snap toothpicks in half and stab the red and green candies together. It should look like Christmas. Now grab two black pipe cleaners and twist them to make a circle. Watch the video at the bottom of this blog post to see how easy it is. Grab two more pipe cleaners and do the same thing, making a wider circle. Now watch how I place the 8 yellow electrons on the electron shells. I don’t actually stab the gumdrops. I show you a trick for how to attach the electrons:









 Now mix the salt and the sand together with a spoon. This is called a mixture. Pour 100ml of water into the salt/sand mixture, and stir for 60 seconds until the salt has dissolved.
Now mix the salt and the sand together with a spoon. This is called a mixture. Pour 100ml of water into the salt/sand mixture, and stir for 60 seconds until the salt has dissolved.




