Today we are hanging some fruit from a chandelier and poking it with a straw–yes, we are measuring the magnitude of force!
Are you ready for a ridiculously fun experiment? You will need the following items: string, a chandelier or door knob, a bendy straw, tape, scissors, a grape, an apple, and a banana.
Here are the results of our experiment:
How to conduct the experiment:
Step 1: Make sure the fruit is completely dry before attempting to tape the string to the fruit. We used packing tape, but apparently flimsy scotch tape also works. I was shocked to see the banana didn’t fall and splat with weak scotch tape holding it up.
Step 2: Tie the other end of the string to the chandelier or door knob.
 This post contains affiliate links. I was compensated for my work in writing this post.
This post contains affiliate links. I was compensated for my work in writing this post.
Step 3: Grab a bendy straw and push each piece of fruit. Notice that the grape does not bend the straw, but the apple and the banana require more force to move, so they bend the straw. The more mass an object has, the more force is required to move it.
The photo above is the moment I discovered the weak tape was holding the banana up. It was quite hilarious, since I was expecting a splat.
See how the grape requires almost no effort to move, since its mass is so small. On the other hand, the other two pieces of fruit require more force to accelerate. To accelerate something means to make it move forward or change its velocity. We accelerated the speed of the fruit in this experiment while determining the magnitude of force required to move each piece.
This fun experiment is from the book Christian Kids Explore Physics by Bright Ideas Press. Why not pick up your own copy today!


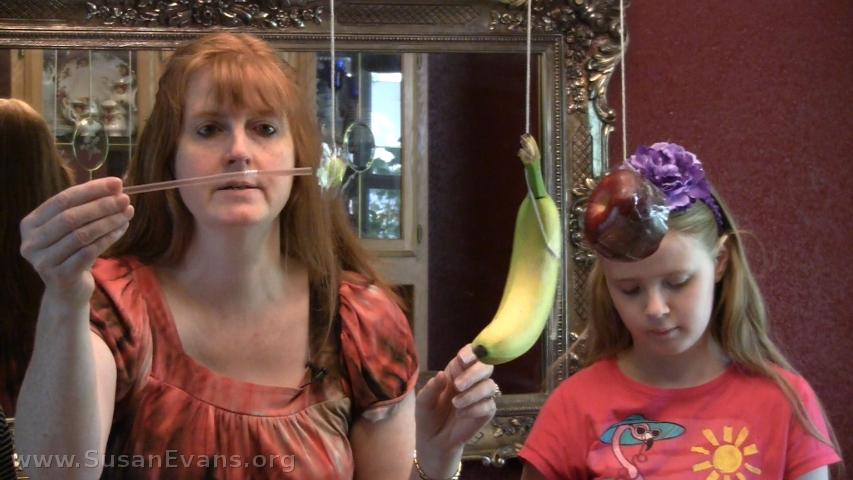
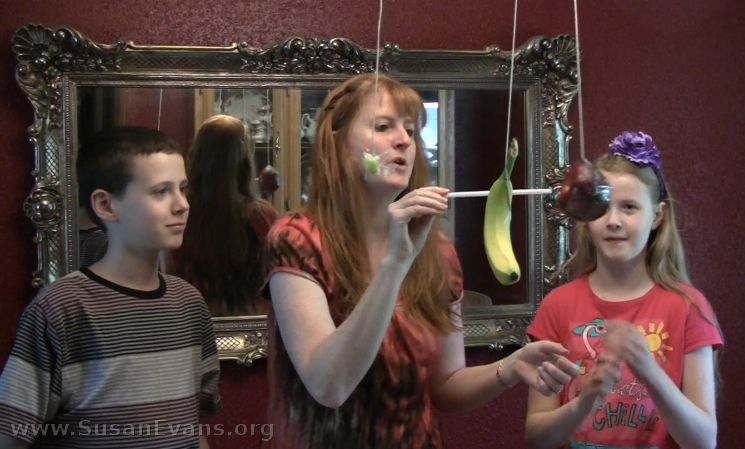









 Now mix the salt and the sand together with a spoon. This is called a mixture. Pour 100ml of water into the salt/sand mixture, and stir for 60 seconds until the salt has dissolved.
Now mix the salt and the sand together with a spoon. This is called a mixture. Pour 100ml of water into the salt/sand mixture, and stir for 60 seconds until the salt has dissolved.




