This post contains affiliate links. I was compensated for my work in writing this post.
Can you teach elementary-level kids something as difficult as chemistry? Yes, you can! This year we studied elementary chemistry, and the curriculum we used was Christian Kids Explore Chemistry by Bright Ideas Press. We had a wonderful time doing all the experiments, which helped us to understand basic chemistry concepts.
The book presented these chemistry concepts in a way that even a child could understand. The science experiments used inexpensive household items, and the experiments were not difficult to perform. My high school kids were present when I went through this elementary-level chemistry book with my two younger kids, and my older kids did better in their high school chemistry because the basic concepts had already been mastered, so the more difficult high school-level concepts were easier to understand.
The multiple choice tests for each chapter were hilarious. One of the answers always made my kids laugh hysterically. You still needed to pay attention and learn all the lessons because it was not possible to pick between the other two possibilities unless you knew the material. In this way, my kids began to look forward to the chemistry tests! Can you believe it? That’s definitely a positive aspect of using this curriculum!
In case you missed any of the experiments that we performed, here is an index of the fun chemistry experiments:
1. Chemistry Tools
2. Filtration Experiment
3. Make Your Own Element Cards
4. Mixtures and Compounds
5. How to Build Atomic Models
6. Atomic Cookies
7. Building Molecular Models
8. Breaking Covalent Bonds
9. Acids and Bases
10. Dissolving Calcium with Acid
11. Measuring the Volume of a Solid
12. Testing Charles’s Gas Law
13. Saltwater Experiment
14. Saturated Solutions
15. Freezing Alcohol
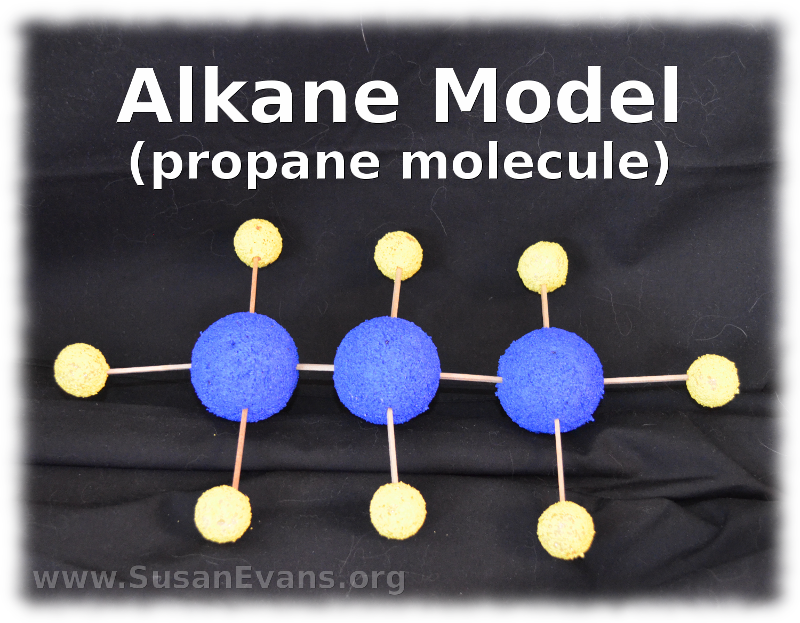
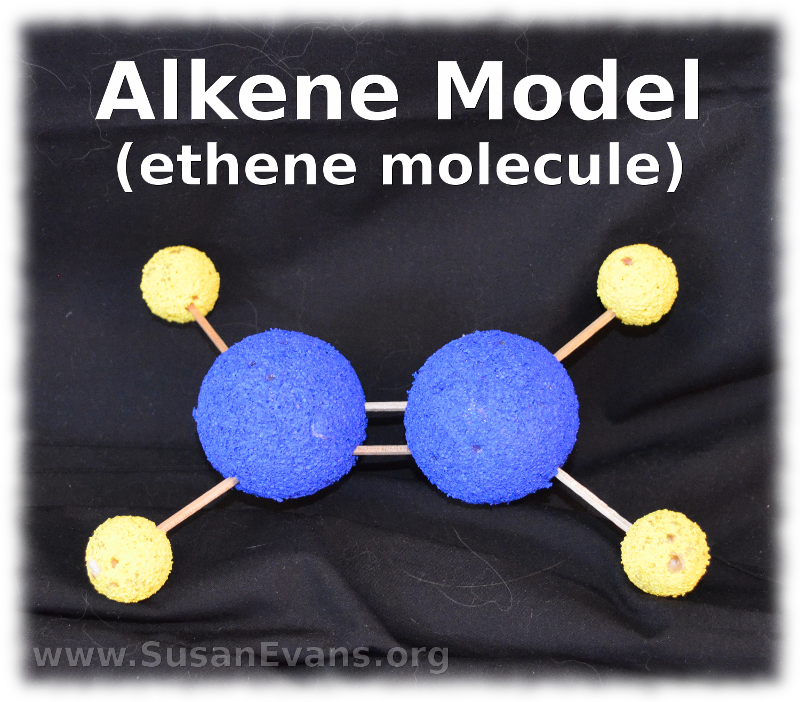
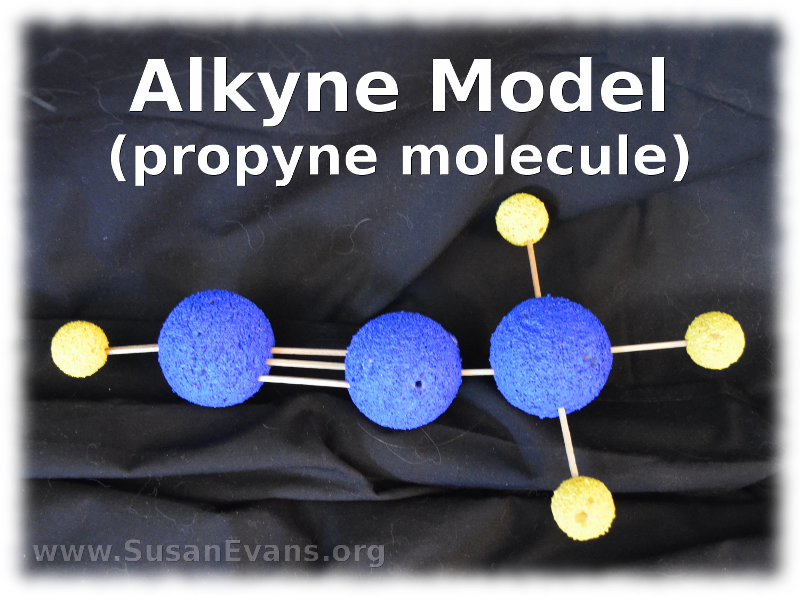
16. Hydrocarbons
I hope you enjoyed all these experiments as much as we did!